Nutropin AQ® [somatropin (rDNA origin) injection]
SOUTH SAN FRANCISCO, Calif. — May 22nd, 2015 — Counterfeiting of Nutropin AQ®
Important Counterfeit Drug Warning
Dear Pharmacist, Health Care Professional and Patient:
Genentech, Inc. recently became aware of the existence in the U.S. of a counterfeit drug product labeled as Nutropin AQ® 10 mg vials in 6-pack boxes. In cooperation with the U.S. Food and Drug administration (FDA), Genentech is informing patients, physicians, pharmacies and wholesalers about this serious health risk. The safety of our patients is of utmost importance and we are cooperating fully with the FDA to investigate this matter and prevent the further distribution of counterfeit product. To our knowledge, the counterfeit product has only been found in distribution in the U.S.
In at least one case the FDA has determined that a vial contained human insulin. In the event that patients have used the counterfeit product they should contact their physician immediately.
The counterfeit product, which is definitely not Nutropin AQ®, was neither manufactured nor distributed by Genentech and may pose a serious health risk to patients. The following Lot Numbers/Expiration Dates have been identified as potential counterfeit drug. Please follow information below to confirm:
Lot No. L9101A4, Expiration May 2002
Lot No. L9504A2, Expiration April 2002
Lot No. L9504A3, Expiration December 2001
The following information may help in determining if the product you have is counterfeit or actual Nutropin AQ® manufactured and distributed by Genentech. Pharmacists, all other health care providers and patients should examine the product before use.
Below are a list of features to compare counterfeit product with authentic Nutropin AQ® with pictures illustrating these differences.
Physicians, please convey this information to your staff and any others who prescribe this product so you can all help alert patients. If you, your staff, or your patients suspect that you are in possession of any counterfeit product, please contact your dispensing pharmacy or contact the FDA Drug Information Branch, Center For Drugs, at (301) 827-4570.
Distributors, if you receive any product that you suspect is counterfeit, quarantine it, store it under labeled conditions, and promptly contact the FDA Division of Drug Information at (301) 827-4570.
Differentiating Packaging Features to Identify Counterfeit Product
Features of Counterfeit Product
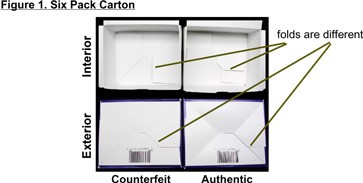
Six Pack Carton:Figure 1
- Inside folding of the carton is different
Features of Authentic Nutropin AQ®
Six Pack Carton:Figure 1
- Bottom outside back flap extends to two corners of the box (there are distinct differences).
Features of Counterfeit Product
Patient Insert:
- Regular stock paper is whiter and thicker.
- Dimensions are 11" x 7", unfolded.
- Color of brand name flash is darker
- Bar code measure is 18 mm.
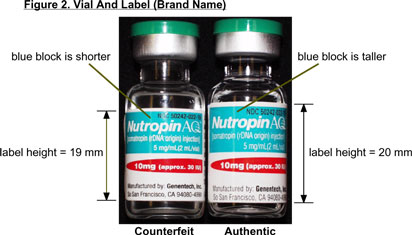
Vial Label:Figures 2 & 3
- Font (type) of lot number and Expiration date is different, with numbers zero, 2, 4, 5, 9 rounded.
- Print is darker and thicker.
- Height (width) of label is approximately 19 mm.
- Blue and red product blocks are shorter.
Features of Authentic Nutropin AQ®
Patient Insert:
- Paper is off-white, thin, light-weight.
- Dimensions are 11.4" x 7", unfolded.
- Color of brand name flash is lighter.
- Bar code is wider and measures 20 mm.
Vial Label:Figures 2 & 3
- Font (type) of letters and numbers of the lot and expiration date is squared.
- Print is crisp and lighter.
- Height (width) of label is approximately 20 mm.
- Blue and red product blocks are taller.
Features of Counterfeit Product
Vial:Figure 3
- Shoulder is more square and higher.
- Width of vial neck is approximately 10.5 mm.
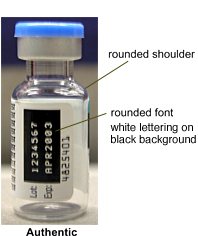
Features of Authentic Nutropin AQ®
Vial:Figure 3
- Shoulder is more rounded.
- Width of vial neck is approximately 9.9 mm.

Features of Counterfeit Product
Product Solution:
- For lot number L9504A2, solution is cloudy at room temperature but clear when refrigerated.
Flip Cap:
- Underside of flip cap is smooth.
Aluminum Seal:Figure 4
- After removing the flip cap, the top surface of the aluminum seal covering the stopper is indented with smooth even metal edge.
Features of Authentic Nutropin AQ®
Product Solution:
- Solution is clear.
Flip Cap:
- Underside of flip cap has 6 notches.
Aluminum Seal:Figure 4
- After removing the flip cap, the top surface of the aluminum seal covering the stopper is indented with 6 dents on the metal edge.

Features of Counterfeit Product
Stopper:Figure 5
- Portion of stopper inserted into the vial has an extra shoulder.
Features of Authentic Nutropin AQ®
Stopper:Figure 5
- Portion of stopper inserted into the vial is smooth.

Other Contact Information FDA Media Contact: Laura Bradbard (301) 827-6252
Genentech Customer Service Department: (800) 551-2231
Genentech Media Contacts: Marie Kennedy (650) 225-8751 or Sabrina Johnson (650) 225-2742
Nutropin AQ® is used for the treatment of growth failure due to inadequate endogenous growth hormone secretion in children and is self-administered daily through subcutaneous injection.
About Genentech
Founded more than 40 years ago, Genentech is a leading biotechnology company that discovers, develops, manufactures and commercializes medicines to treat patients with serious and life-threatening medical conditions. The company, a member of the Roche Group, has headquarters in South San Francisco, California. For additional information about the company, please visit http://www.gene.com.