News Features
Access media kits and related information for our featured news stories.
65 results

10 Oct 2024 FDA Approves New Targeted Treatment For Advanced Hormone Receptor-Positive, HER2-Negative Breast Cancer With A PIK3CA Mutation ItovebiOn October 10, 2024, the FDA approved Genentech’s medicine for PIK3CA-mutated HR+, HER- advanced breast cancer. |
13 Sep 2024 FDA Approves the First and Only Twice-A-Year 10-Minute Subcutaneous Injection for People with Relapsing and Progressive Multiple Sclerosis Ocrevus ZunovoOn September 13, 2024, the FDA approved Genentech’s subcutaneous medicine for both relapsing and progressive multiple sclerosis in adults. |

16 Feb 2024 FDA APPROVES THE FIRST AND ONLY MEDICINE FOR CHILDREN AND ADULTS WITH ONE OR MORE FOOD ALLERGIES XolairOn February 16, 2024, the FDA approved the first and only medicine to reduce allergic reactions in people with one or more IgE-mediated food allergies. |

15 Jun 2023 FDA Approves Genentech’s Fixed-Duration Bispecific Antibody for Relapsed/Refractory Diffuse Large B-Cell Lymphoma ColumviOn June 15, 2023, the FDA approved Genentech’s medicine for relapsed and refractory diffuse large B-cell lymphoma. |

19 Apr 2023 FDA Approves Genentech’s Medicine For People With Certain Types of Previously Untreated Diffuse Large B-Cell Lymphoma PolivyOn April 19, 2023, the FDA approved Genentech’s medicine for certain types of previously untreated DLBCL. |

22 Dec 2022 FDA Approves Genentech’s First-in-Class Medicine for Relapsed/Refractory Follicular Lymphoma After at Least Two Prior Therapies LunsumioOn December 22, 2022, the FDA approved Genentech’s medicine for advanced follicular lymphoma. |
28 Jan 2022 FDA Approves Genentech’s Bispecific Antibody For Two Leading Causes Of Vision Loss VabysmoOn January 28, 2022, the FDA approved Genentech’s bispecific antibody for wet age-related macular degeneration (AMD) and diabetic macular edema (DME). |
22 Oct 2021 FDA Approves Genentech’s New Treatment For Wet Age-Related Macular Degeneration (AMD) SusvimoOn October 22, 2021, the FDA approved Genentech’s treatment for wet AMD, a leading cause of blindness. |
15 Oct 2021 FDA Approves Genentech’s Immunotherapy For People With Certain Types Of Early-Stage Lung Cancer TecentriqOn October 15, 2021, the FDA approved our immunotherapy for adjuvant treatment of certain types of lung cancer |
21 Oct 2020 FDA Grants Full Approval For Genentech’s Acute Myeloid Leukemia Medicine Under the FDA’s Real-Time Oncology Review Pilot Program And Project Orbis Initiative VenclextaOn October 16, 2020, the FDA granted full approval for our acute myeloid leukemia (AML) medicine. |

14 Aug 2020 FDA Approves Genentech’s Treatment for Neuromyelitis Optica Spectrum Disorder (NMOSD) EnspryngLearn more about the recent FDA approval of our neuromyelitis optica spectrum disorder (NMOSD) medicine. |

7 Aug 2020 FDA Approves Genentech’s Treatment for Spinal Muscular Atrophy (SMA) in Adults and Children 2 Months and Older EvrysdiLearn more about the recent FDA approval of our spinal muscular atrophy (SMA) medicine. |

10 Jun 2019 Genentech Medicine Received Accelerated FDA Approval as a New Treatment Option for People with Previously Treated Aggressive Lymphoma PolivyOn June 10, 2019, the FDA granted accelerated approval for a new Genentech medicine in combination with bendamustine plus Rituxan (rituximab) for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma who have received at least two prior therapies. |
15 May 2019 FDA Approves New Fixed-Duration Treatment Option for Previously Untreated Chronic Lymphocytic Leukemia VenclextaOn May 15, 2019, the FDA approved a fixed-duration, chemotherapy-free combination of Genentech medicines for people with previously untreated chronic lymphocytic leukemia. |
3 May 2019 FDA Approves New Adjuvant Treatment for Certain Patients with HER2-Positive Early Breast Cancer KadcylaOn May 3, 2019, the FDA approved a Genentech medicine for adjuvant (after surgery) treatment of people with HER2-positive early breast cancer (EBC) who have residual invasive disease after neoadjuvant (before surgery) taxane and Herceptin-based treatment. |

18 Mar 2019 FDA Approves First and Only Cancer Immunotherapy for Initial Treatment of Difficult-to-Treat Type of Lung Cancer TecentriqOn March 18, 2019, the FDA approved Genentech’s immunotherapy, in combination with chemotherapy (carboplatin and etoposide), for the initial (first-line) treatment of adults with extensive-stage small cell lung cancer. |

8 Mar 2019 Genentech Medicine FDA Approved as First Cancer Immunotherapy for a Type of Breast Cancer TecentriqOn March 8, 2019, the FDA granted accelerated approval to a Genentech medicine for the treatment of adults with unresectable locally advanced or metastatic triple-negative breast cancer in people whose tumors express PD-L1, as determined by an FDA-approved test. |

24 Jan 2019 Genentech Announces Alexander Hardy As Chief Executive OfficerGenentech, a member of the Roche Group, today announced the appointment of Alexander Hardy to chief executive officer, effective March 1, 2019. |

6 Dec 2018 FDA Approves New Immunotherapy Combination for a Specific Type of Metastatic Lung Cancer TecentriqOn December 6, 2018, the FDA granted approval to a new immunotherapy combination for the initial treatment of people with metastatic non-squamous non-small cell lung cancer with no EGFR or ALK genomic tumor aberrations. |
21 Nov 2018 FDA Approves New Treatment Option for People Newly-Diagnosed With Acute Myeloid Leukemia VenclextaOn November 21, 2018, the FDA approved a Genentech medicine, in combination with azacitidine, or decitabine, or LDAC, for people newly-diagnosed with AML who are age 75 years or older, or who are ineligible for intensive chemotherapy due to coexisting medical conditions. This indication is approved under accelerated approval based on response rates. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. |

24 Oct 2018 FDA Approves New Single-dose Treatment For Influenza XofluzaOn October 24, 2018, the FDA approved the first and only single-dose, oral antiviral medicine to treat the flu. |
4 Oct 2018 FDA Approves New Prophylactic Treatment For Hemophilia A Without Factor VIII Inhibitors HemlibraOn October 4, 2018, the FDA approved a Genentech medicine as a new prophylactic (preventative) treatment for adults and children, ages newborn and older, with hemophilia A without factor VIII inhibitors, making it the only approved medicine for hemophilia A with and without factor VIII inhibitors that can be self-administered under the skin (subcutaneously) once weekly, every two weeks or every four weeks. |
13 Jun 2018 FDA Approves Genentech’s Avastin® (Bevacizumab) Plus Chemotherapy as a Treatment for Women with Advanced Ovarian Cancer Following Initial Surgery AvastinOn June 13, 2018 the FDA approved a Genentech medicine for the treatment of women with advanced (stage III or IV) epithelial ovarian, fallopian tube or primary peritoneal cancer following initial surgical resection. |
8 Jun 2018 FDA Approves New Treatment Option for Previously Treated Chronic Lymphocytic Leukemia VenclextaOn June 8, 2018, the FDA approved a Genentech medicine for people with previously treated chronic lymphocytic leukemia. |
20 Dec 2017 FDA Approves New Treatment Option for Adjuvant Treatment of Specific Type of Early Breast Cancer PerjetaOn December 20, 2017, the FDA approved a Genentech medicine for adjuvant (after surgery) treatment of HER2-positive early breast cancer at high risk of recurrence. |
16 Nov 2017 FDA Approves New Treatment Option for Previously Untreated Advanced Follicular Lymphoma GazyvaOn November 16, 2017, the FDA approved a Genentech medicine as a treatment option for people with previously untreated advanced follicular lymphoma (stage II bulky, III or IV). |

16 Nov 2017 FDA Approves New Once-Weekly Prophylactic Treatment For Hemophilia A With Inhibitors HemlibraOn November 16, the FDA approved a Genentech medicine as a prophylactic (preventative) treatment for adults and children with hemophilia A with factor VIII inhibitors that can be self-administered once weekly by injection under the skin (subcutaneously). |
6 Nov 2017 FDA Approves Genentech Medicine for First-Line Treatment of Specific Type of Lung Cancer AlecensaOn November 6, 2017, the FDA approved a Genentech medicine for the treatment of people with a specific type of lung cancer. |
22 Jun 2017 FDA Approves New Time-Saving Treatment Option for People with Certain Blood Cancers Rituxan HycelaOn June 22, 2017, the FDA approved a Genentech medicine for subcutaneous (under the skin) injection for the treatment of certain blood cancers. |
17 Apr 2017 FDA Approves New Option for Certain People with Advanced Bladder Cancer TecentriqOn April 17, 2017, the FDA granted accelerated approval to a Genentech cancer immunotherapy medicine as an initial treatment for certain people with advanced bladder cancer. |

17 Apr 2017 FDA Approves New Treatment Option for People with Diabetic Retinopathy LucentisOn April 17, 2017, the FDA granted approval to a Genentech medicine for the monthly treatment of all forms of diabetic retinopathy, a potentially blinding eye disease. |
6 Dec 2016 FDA Approves Medicine for Women with Platinum-Sensitive Recurrent Ovarian Cancer AvastinOn December 6, 2016 the FDA approved a medicine for women with platinum-sensitive recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer. |

18 Oct 2016 FDA Approves New Immunotherapy Medicine for a Type of Metastatic Lung Cancer TecentriqOn October 18, 2016, the FDA granted approval to a new cancer immunotherapy medicine for the treatment of a type of metastatic lung cancer. |
16 Sep 2016 Flu Season Resources TamifluOn average, 5-20% of the US population gets the flu each year, and more than 200,000 are hospitalized from seasonal flu related complications. To recognize flu symptoms, remember Flu F.A.C.T.S. (Fever, Aches, Chills, (extreme) Tiredness, and Sudden Onset). |

18 May 2016 FDA Approves New Immunotherapy Medicine for Specific Type of Advanced Bladder Cancer TecentriqOn May 18, 2016, the FDA granted accelerated approval to a new cancer immunotherapy medicine for the treatment of a specific type of advanced bladder cancer. |
11 Apr 2016 FDA Approves New Treatment Option for Hard-To-Treat Type of Chronic Lymphocytic Leukemia VenclextaOn April 11, 2016, the FDA granted accelerated approval to a new medicine for the treatment of a hard-to-treat type of chronic lymphocytic leukemia. |
26 Feb 2016 FDA Approves New Option For Certain People With Previously Treated Follicular Lymphoma GazyvaOn February 26, 2016, the FDA approved a Genentech medicine for people with follicular lymphoma whose disease did not respond to or returned after treatment with another medicine. |

11 Dec 2015 FDA Approves New Treatment Option for Specific Type of Lung Cancer AlecensaOn December 11, 2015, the FDA granted accelerated approval to a new medicine for the treatment of a specific type of lung cancer. |

10 Nov 2015 FDA Approves New Treatment Option for Advanced Melanoma CotellicOn November 10, 2015, the FDA approved a new medicine for the treatment of advanced melanoma. |
6 Feb 2015 FDA Approves Genentech Medicine for Diabetic Retinopathy in People with Diabetic Macular Edema LucentisOn February 6, 2015, the FDA approved a Genentech Medicine for the treatment of diabetic retinopathy in people with diabetic macular edema (DME). |
14 Nov 2014 FDA Approves Genentech Medicine for Women with Platinum-Resistant Recurrent Ovarian Cancer AvastinOn November 14, 2014, a Genentech medicine received FDA approval for the treatment of women with platinum-resistant, recurrent, epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have received no more than two prior chemotherapy regimens. |

15 Oct 2014 FDA Approves Medicine for Idiopathic Pulmonary Fibrosis EsbrietOn October 15, 2014, the FDA approved a medicine for the treatment of idiopathic pulmonary fibrosis (IPF). |
14 Aug 2014 FDA Approves Genentech Medicine for Women with Advanced Cervical Cancer AvastinOn August 14, 2014, a Genentech medicine received FDA approval for use in persistent, recurrent or metastatic cervical cancer. It is the first biologic medicine approved in combination with chemotherapy to help women with this type of cancer live longer than with chemotherapy alone. |
1 Jul 2014 Genentech Announces Definitive Agreement to Acquire Seragon PharmaceuticalsOn July 1, 2014, Genentech entered into an agreement to acquire Seragon Pharmaceuticals, Inc., a privately held biotechnology company based in San Diego, California. |
21 Mar 2014 FDA Approves Xolair® (omalizumab) for Subcutaneous Use for People with Chronic Idiopathic Urticaria (CIU), a Form of Chronic Hives XolairOn March 21, 2014, the FDA approved a Genentech medicine for people 12 years of age and older for the treatment of chronic idiopathic urticaria (CIU), a form of chronic hives. |

5 Dec 2013 ASH 2013: Press Releases, Fact Sheets & MoreWe're presenting important new findings on unapproved and investigational medicines at ASH 2013. Read press releases and learn about our presence at ASH this year. |
1 Nov 2013 FDA Approves New Genentech Medicine for Chronic Lymphocytic Leukemia (CLL) GazyvaOn November 1, 2013, the FDA approved a new Genentech medicine for people with previously untreated CLL. It was the first medicine approved with the FDA’s Breakthrough Therapy Designation. |

15 May 2013 2013 ASCO Annual MeetingGenentech to report new advances at the American Society of Clinical Oncology Annual MeetingGenentech will present important new data from studies of several cancer medicines at the 49th Annual Meeting of the American Society of Clinical Oncology (ASCO) from May 31-June 4, 2013, in Chicago.This page is intended to be a media resource for Genentech developments at ASCO. It will be updated frequently throughout the meeting. |
14 May 2013 FDA Approves First Personalized Medicine for EGFR Mutation-Positive Metastatic Non-Small Cell Lung Cancer in the United StatesOn May 14, 2013, the FDA approved a personalized medicine for people newly diagnosed with a genetically distinct type of metastatic NSCLC. |

10 May 2013 Celebrating the 60th Anniversary of Watson & Crick’s DiscoveryGenentech built an iPad app that turns a primary genetic concept into a maddeningly addictive mobile game. |
23 Jan 2013 FDA Approves New Use of Avastin Plus Fluoropyrimidine-Based Chemotherapy in Metastatic Colorectal Cancer AvastinOn January 23, 2013, a Genentech medicine received FDA approval for a new use in metastatic colorectal cancer. |

12 Oct 2012 FDA Approves Expanded Indication for Actemra in RA ActemraOn October 12, 2012, a Genentech medicine received approval from the FDA to expand its indication for Rheumatoid Arthritis. |

25 Aug 2012 Genentech's gRide Program Achieves Landmark MilestonegRide, Genentech's employee commuting program, has saved 100 million miles of driving since the program's inception began in late 2006. The program began as a way to reduce the number of vehicles that travel to and park on the South San Francisco campus. |
|
10 Aug 2012 Lucentis Approved for Treatment of Diabetic Macular Edema (DME) LucentisOn August 10, 2012, a Genentech medicine was approved by the FDA for diabetic macular edema (DME). |
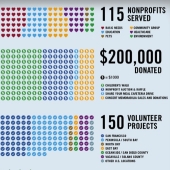
11 Jun 2012 Genentech Gives Back Week 2012Genentech Gives Back Week (June 11 - 16, 2012) is a unique opportunity for Genentech employees to support the communities where we live and work. |
8 Jun 2012 Perjeta Approved For HER2-Positive Metastatic Breast Cancer PerjetaOn June 8, 2012, a Genentech medicine was approved by the FDA for previously untreated HER2-positive metastatic breast cancer. |
|
30 May 2012 Genentech at ASCO 2012Genentech will present important new data on several cancer medicines at the 48th Annual Meeting of the American Society of Clinical Oncology (ASCO). |

17 May 2012 Landmark Alzheimer's Prevention TrialGenentech, the Banner Alzheimer's Institute, and the National Institutes of Health are collaborating on the first-ever prevention trial in cognitively healthy individuals who are likely to develop Alzheimer's disease due to their genetic history. |

30 Jan 2012 Erivedge Approved For Advanced Basal Cell Carcinoma ErivedgeOn January 30, 2012, a Genentech medicine was approved by the FDA for the treatment of adults with a type of skin cancer, called basal cell carcinoma (BCC), that has spread to other parts of the body or that has come back after surgery or that their healthcare provider decides cannot be treated with surgery or radiation. |

17 Aug 2011 Genentech Medicine Approved For Certain Type Of Melanoma ZelborafOn August 17, 2011, a Genentech medicine was approved by the FDA for a type of inoperable or metastatic melanoma. |
20 Oct 2010 Genentech Medicine Approved in Stomach Cancer HerceptinOn October 20, 2010, a Genentech medicine was approved by the U.S. Food and Drug Administration (FDA) for stomach cancer. |
|
18 Oct 2010 25th Anniversary of First Product ApprovalOctober 18, 2010 is the 25th anniversary of Genentech's first product approval and the first recombinant biotech drug to be manufactured and marketed by a biotechnology company. |
|
21 Sep 2010 Napoleone Ferrara Named 2010 Lasker Award WinnerGenentech Fellow Napoleone Ferrara is the winner of the 2010 Lasker Award for clinical research, presented annually to an investigator "whose contributions have improved the clinical treatment of patients." |
|
13 Sep 2010 Genentech Goes to Town 2010Genentech Goes to Town takes place September 13 to September 24 in South San Francisco, Vacaville, Oceanside and Hillsboro. The Genentech Goes to Town program supports our communities by providing employees with special "GenenMoney" to spend at local businesses. |

14 Jun 2010 Genentech Gives Back Week 2010Genentech Gives Back Week voluntary activities each day that help Genentech employees collectively make a difference in our communities. All employees are invited to participate in Genentech Gives Back as their time and interest allows. |
